William H. Calvin and George A. Ojemann, Inside the Brain: Mapping the Cortex, Exploring the Neuron (New American Library, 1980), chapter 5. See also http://WilliamCalvin.com/ Bk1/bk1ch5.htm. |
William H. Calvin
University of Washington |

|
5 Deep Inside the Brain: Compensating for Parkinson's Disease |
©1980 by William H. Calvin and George A. Ojemann |
Down in the OR, the surgical team works in relative silence. The staff in the OR may not be as quiet or as reverent as a concert audience, but it is far quieter than the crowd in the glassed-in gallery, whose voices cannot be heard in the OR unless the intercom button is pressed. The visibility from the brightly lit OR into the darkened gallery is also poor, so the crowd can engage in wisecracks and imitations of the surgeons without serious chance of discovery. "Is this the first time you've seen a living, working brain," the neurologist asks one of the medical students standing near the EEG machine. "Yes, though I could see it better if the surgeon were transparent," complains the student, contemplating the surgeon's back. "I always thought brains were gray and hard. Turns out they are pink and soft. And look at the size of those blood vessels on the surface!" "Well, the brain does get one-fifth of everything that the heart pumps," comments the second student. "Got to carry it in something." "That's the middle cerebral artery in the center of the craniotomy opening," notes the neurologist, pointing. "What happens if it gets plugged up?" The students recognize the standard faculty teaching gambit, the rhetorical question. They respond by reciting to her the whole story of the middle cerebral artery, how it serves the speech and language areas of the brain, and they then rattle off the different kinds of aphasia which can result from blockage of it. "That's a nice job of covering the surface of the brain, but can you think of any areas in the depths of the brain concerned with speech?" she asks. "Well, the thalamus must have something to do with speech," ventures the first medical student.' "They presented a patient with Parkinson's disease in the neurology rounds the other day. He had an operation on his thalamus and was aphasic for several weeks after the surgery." "Yes, but lots of Parkinson's patients have trouble talking," points out the second student in a dubious tone of voice. "Their voices fade out when they talk on the phone and-" "Whoa!" interrupts the neurologist. "Suppose you first tell me about the Parkinson's-disease patient. Then we'll discuss treatments for Parkinsonism, and then come back to the aphasia. OK?" The neurologist is off on another teaching tangent. "This patient said that he couldn't get up out of a soft chair," the first student recalls, "and sometimes couldn't roll over in bed, or cut steak with a knife and fork. He would get stuck in one position and had a hard time getting going again. I guess that he had fallen down several times, too." "And . . . ?" "He had a tremor of his hands-no, it was only his left hand-that was most noticeable when he was resting. If you moved his left arm for him, it felt very stiff and would jerk a little -- that's 'cogwheel rigidity.' And of course he couldn't move his fingers very fast, and he didn't move his face much, either. His face looked like a mask, just like the textbooks say. His voice kept getting softer and softer as he talked to us. When we asked him to walk around the room, he took little steps and seemed ready to topple over -- just as if the top of him was trying to go faster than his legs." "And what treatments had he had besides the surgery?" queries the neurologist. "He's currently on levodopa." And, except for the drug called levodopa and the mention of thalamic surgery, the students' description of the disease is very much the same as that given by James Parkinson in 1817 in his Essay on the Shaking Palsy. As so often happens in medicine, it is simpler to identify a disease with a person's name than it is to give it a more meaningful (but longer) label. For nearly a century and a half, the treatment of Parkinson's disease changed little, although cases became more common. Cases of Parkinson's disease were more frequent following the influenza epidemic after the First World War, particularly in those people who suffered a severe case of encephalitis, By trial and error, some drugs were found which helped some victims, but their side effects prevented many patients from taking them. At the beginning of the space age, many Parkinson's victims were almost as bad off as those at the beginning of the industrial revolution had been. Many basic science discoveries, however, had occurred in those 150 years which provided the basis for a major advance in the therapy of Parkinson's disease. Anatomically, the brain had progressed from being terra incognita to being well labeled. Understanding the function of those hundreds of anatomical subdivisions is a much harder task, and the success story with Parkinson's disease illustrates how progress is made. The part of the brain seen in most brain maps is the cerebral cortex. It is a layer of nerve cells about 3 mm thick forming an outer shell. Something else - must occupy the rest of the brain's thickness. First of all, the surface layer is infolded rather like the skin of a green pepper, except much more so. This infolding increases the total surface area of cerebral cortex many times in the human brain; some mammalian brains, such as those of the rabbit and some New World monkeys, have very little infolding. Below the surface layer of nerve cells, there is a lot of "white matter." These are the axons of nerve cells entering and leaving the cerebral cortex. Their white appearance is due to the color of the insulation on the axons, called myelin. The white matter contains these insulated axons and the cells that support them and hold them together, called glia ("glue" in Greek). The white matter is thus analogous to a bundle of white cables running from one part of a computer to another. Gray matter is the name for the other areas, such as cerebral cortex, where insulated axons are not the overwhelming ingredient. In preserved brain, the color is grayer than that of the white matter"; in a living brain, "gray" matter is really a pinkish brown. Not only are there lots of cell bodies, dendrites, and synapses in gray matter, but there is a richer blood supply too, which contributes to the non-gray color.
Deep in the white matter are other collections of gray matter, the nerve cells of the striatum (also called the basal ganglia). Still deeper, where the two cerebral hemispheres join with the long core of the brain (the brain stem), lies another large cluster of nerve cells collectively called the thalamus. Beneath the thalamus is a narrow band of nerve cells whose color is much darker than the usual gray, due to a pigment derived from the neurotransmitter dopamine contained within the cells. This group of nerve cells is called the substantia nigra ("black substance" in Latin), and their axons terminate in the striatum. As we shall see, they are among the nerve cells which are destroyed by Parkinson's disease. During the twentieth century, the electrical and chemical nature of nerve cells has become apparent. Different nerve cells specialize in the production of different neurotransmitter substances. Those in one part of the substantia nigra, for instance, happen to produce dopamine, which they release at the synapse in response to electrical signals such as impulses. In addition to transmitting one type of neurotransmitter, each nerve cell receives several different neurotransmitters from upstream nerve cells. Some of these neurotransmitters produce an excitatory effect upon the nerve cell; other neurotransmitters may antagonize, or inhibit, that cell. The push and pull is carried out by balancing the voltage of the nerve cell, the excitatory inputs raising the voltage and the inhibitory ones lowering it. Drugs may affect the process at a number of steps. For example, the amount of neurotransmitter available for release by impulses may be changed by drugs. The second student, who has been looking through binoculars at the convolutions on the surface of Neil's exposed brain, several meters (six feet) away on the other side of the glass, turns and says, "I read somewhere about an autopsy study on the brains of Parkinson's-disease patients. I think it showed that lots of dopamine-containing neurons in the substantia nigra were missing, but no one has ever really told us the story behind Parkinson's." "All right," says the neurologist. "I'll give you the three-minute capsule summary. In 1957, Carlsson and his co-workers were experimenting on animals with a drug which caused a massive loss of dopamine from the striatum. Their animals were rigid and moved around very slowly, just like Parkinson's patients. Then in 1960 came that autopsy study that you had heard about, in which Ehringer and Hornykiewicz found that dopamine was missing from the striatum of Parkinson's patients. "What is now obvious is that the dopamine in the striatum is the transmitter substance released from the axons of neurons which come from the substantia nigra. One of the reasons that there's so little dopamine in the striatum is that a lot of the substantia nigra cells have died. Now, you can apparently get the remaining cells to produce extra dopamine by providing them with more of the raw material from which they make dopamine -- that's the theory behind giving patients massive doses of levodopa, which is the molecule from which dopamine is created. But why do the neurons in the substantia nigra die?" The neurologist poses one of her favorite teaching questions.
The second student looks thoughtful. "Some cases occurred right away during that great influenza epidemic after the First World War. And lots of cases that you see today are also people who suffered a really bad case of the flu way back when. But their symptoms didn't appear for many years. If the flu killed off those substantia nigra neurons, why doesn't the disease always start at the same time as the flu attack? Why thirty to fifty years later? Does the virus hang around, slowly killing neurons as in one of those degenerative diseases?" "A good question," replies the neurologist. "A slow virus is one possibility. But it turns out that normal people - you and me, presumably - are always losing neurons in the substantia nigra every day. It's a higher percentage than the average for the whole brain. In substantia nigra the total declines by half before seventy years of age. Presumably, the remaining neurons compensate for this loss of their neighbors. But perhaps there is a minimum number of cells that you've got to have in the substantia nigra, perhaps twenty percent of what you were born with, or the compensation fails. Fewer than that, and you get stiff, develop the shakes, and so on." "So maybe the flu killed off half the neurons back in 1919, but it doesn't cause any trouble until a lot more die naturally and you reach the twenty-percent level, or whatever," exclaims the first student. "That's the theory. Or at least, that's one of the theories floating around, waiting to be tested. But essentially, there's a group of neurons which are overactive because many of the neurons which inhibit them have died." "Why not compensate by just shutting down some of the excitatory inputs which increase activity in the striatum? If there's less inhibition, why not less excitation? Wouldn't that balance things?" asks the first student.
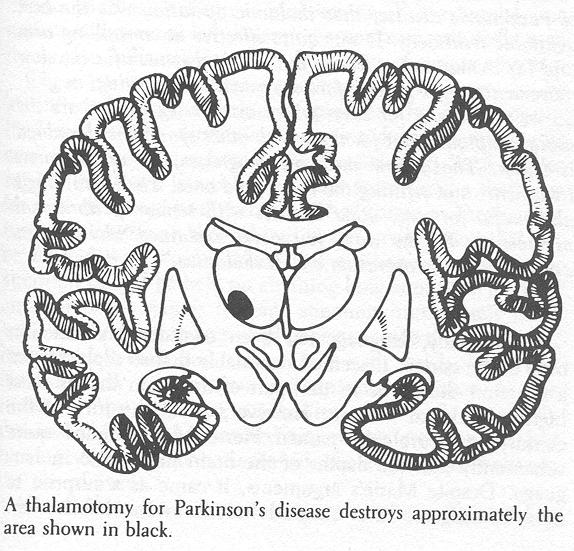
"Good idea. Actually, there are opportunities all along the chain of neurons because, at each. neuron, you have both excitatory and inhibitory inputs. Some push backward and others pull. Presumably, that's how the surgical treatments for Parkinson's disease have their effect. Neurons deep in the thalamus are destroyed in such operations, either by overheating them or by freezing them. We're not sure exactly what role those neurons play, but there's good evidence that they inhibit other neurons in the chain of neurons controlling muscle tone, so their loss helps restore the balance between push and pull. I think you said earlier that this patient had such an operation. " "Yes, and he was aphasic for a while afterward. It was done in left brain." "Any changes in his motor function after that operation?" "Well, he said that he used to shake more in the right arm and it was much stiffer than the left. But in the ten years since that operation, the right arm doesn't shake at all. The left arm tremor has gotten worse, though, and his face more masklike. Does anyone do operations like that anymore?" "In the decade before the discovery of levodopa as a treatment of Parkinson's disease, that thalamic operation was the best available treatment. It was quite effective at controlling arm and leg tremor and stiffness, but didn't do much for the slow movements, voice fading, face movement, swallowing, or gait. So we still use it for cases where arm or leg tremor are the main symptoms. But for those with other symptoms, levodopa is better. That's how medicine progresses, by adding new treatments and refining the uses of old ones. The operation is also useful for some other diseases with tremor or abnormal movements. By the way, you never explained why he was aphasic after the operation on his thalamus." Traditionally, language has been considered a function only of the cortex. It seemed reasonable that so sophisticated a function should be in that part of the brain that is most highly developed in man. However, about the turn of the century, a neurologist named Pierre Marie argued quite vehemently that the depths of the brain had a role in language. Despite Marie's arguments, it came as a surprise to most investigators that surgical lesions in the left human thalamus sometimes led to a language disturbance. Usually this was a temporary difficulty in naming things, but occasionally it was permanent. Equally surprising were observations of altered language after small hemorrhages confined to the left thalamus. Such observations were made only after the development of diagnostic techniques that allowed the identification of this type of damage during life. Large thalamic hemorrhages destroy so many structures that patients are in coma, their language function untestable. Smaller hemorrhages weren't regularly diagnosed until techniques like the computerized tomographic (CT) scan came along. CT scan clearly shows small thalamic hemorrhages and, in those cases with left thalamic hemorrhages, a new pattern of language disturbance has been identified: there is fluent speech (as in Wernicke's-area cortical damage), but the same word is repeated over and over (as in Broca's-area cortical damage), and there are wide swings in performance, from nearly normal to unintelligible. Wildly inappropriate words appear in these patients' conversations. For example, one patient with a small left thalamic hemorrhage repeatedly used 2 the phrase "affirmative action" for names of simple objects. These left thalamic areas concerned with language can be identified during the thalamic operations for Parkinsonism (thalamotomy) by stimulation mapping during naming, just as cortical areas have been in Neil's operation. Such studies suggest that there is a common mechanism in the thalamus for both language and memory. That common mechanism focuses attention on those things in the environment that can be labeled with words. When this mechanism is damaged, attention cannot be held on anything long enough to produce an appropriate name. Instead, attention immediately shifts and a random extraneous word pops out. Like the left cortex, only the left thalamus is concerned with words. The right thalamus has an attention mechanism for shapes. In the thalamus the attention mechanism for words is clearly dominant. When it is turned on, shapes are ignored. But turning on the attention mechanism for shapes doesn't change the use of words. This thalamic attention mechanism can be manipulated by electrical stimulation, with most unusual results. If the stimulating current is applied to the thalamus when information is coming in, that information will be remembered, seconds to days later, about twice as accurately as similar information coming in without stimulation. If the same current is applied when information already in memory is to be retrieved, retrieval is faster than usual but with many more errors. This attention mechanism seems to regulate what comes into or out of memory at any given moment, almost as if it were opening or closing a gate. When something is coming in, similar information cannot simultaneously be retrieved from memory. The attention mechanism also determines how easily something coming in will later be remembered. Most inputs have both verbal (word label) and spatial features. The relative strength of this attentional mechanism may determine how many of those verbal or spatial features are remembered, and the number and types of associations available from memory to relate to this new input. Enhancing memory for incoming information by thalamic stimulation may eventually become an aid in rapidly retraining stroke patients with language disorders. One can imagine science-fiction uses, too-for example, helping medical students to study for exams. 
"But doesn't this complication of aphasia make the thalamic operation too risky as a treatment for Parkinson's disease?" one of the students asks. "Fortunately, the aphasia is usually transient, disappearing in a few days," the neurologist remarks as she turns to prepare her EEG machine. "But there is a small risk of persisting aphasia and other neurologic deficits with that operation. And, for that matter, with the one going on here today- a few chances in a hundred in each case. But there are risks to all treatments, you know, whether drugs or surgery. Levodopa carries risks, too. Medicine is always a process of balancing off these risks against the chances of helping. No operation or drug is risk-free, though Congress and the Food and Drug Administration seem to forget that sometimes."
|
 Continue to CHAPTER 6
Continue to CHAPTER 6
Notes and
References for this chapter Copyright ©1980 by |